Studies Using Checkpoint Modifiers
Virion Therapeutics is a clinical-stage biotechnology company developing novel T-cell-based immunotherapies. They utilize proprietary genetically encoded checkpoint modifiers to enhance and broaden CD8+T-cells responses.
Dr. Andrew Luber, CEO of Virion, commented, “The initiation of our first clinical trial, for VRON-0200, represents a major milestone in Virion’s mission of bringing innovative immunotherapies to patients with cancer and chronic infectious diseases. Enhancing and expanding a patient’s own immune responses, by targeting T cell activation, via checkpoint modification, is unique to Virion, and these first data in humans will provide useful information for our proprietary platform technologies and pipeline, including VRON-0300, which is in development for patients with advanced solid tumors.”
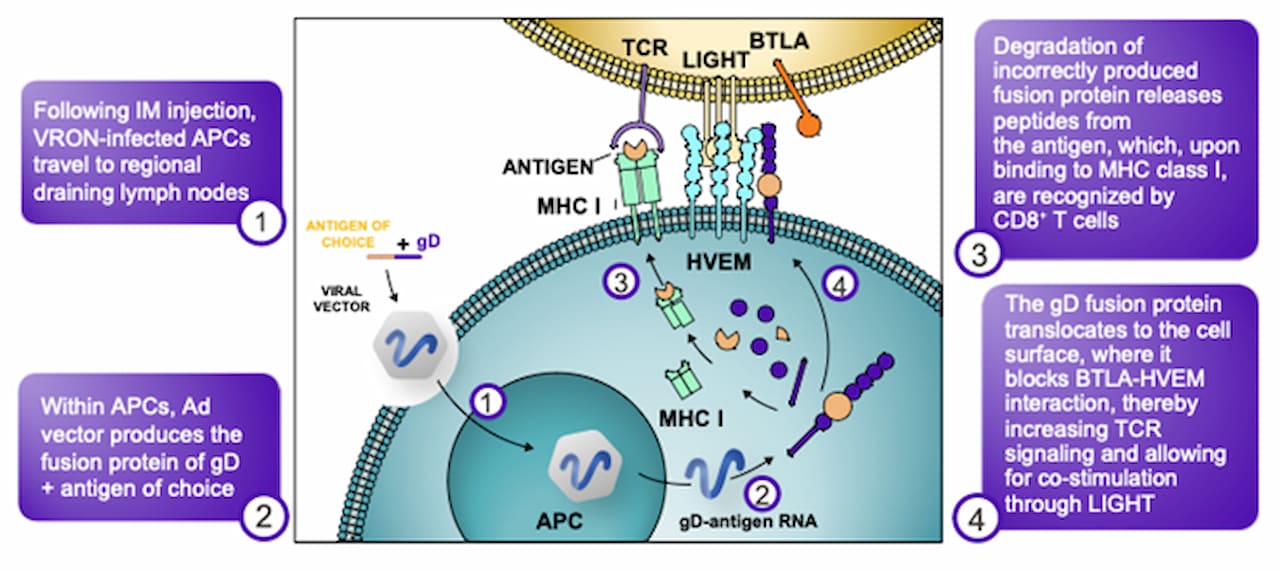
gD BTLA-HVEM Blockade Enhances and Broadens T Cell Activation (www.VirionTx.com)
(Source: Ocean Biomedical, Inc.)
“These clinical milestones and new data have generated a high level of interest from potential stakeholders and industry partners, for both our VRON-0200 and VRON-0300 programs,” Luber added.
Preclinical studies using checkpoint modifiers have shown consistent and extraordinary immune responses and clinical activity in different diseases. In addition to the recently presented oncology data, Virion has recently begun enrolling its Phase-1b clinical trial for persons with chronic hepatitis B virus.
VRON-0200 for HBV
VRON-0200 is being developed with the goal of providing a functional cure for a disease that affects more than 300 million patients worldwide. It is designed to help overcome a key cause of chronic hepatitis B virus, immune exhaustion, by stimulating a patient’s own immune response to help control the infection. Virion believes that this novel mechanism using checkpoint modifiers is a different approach to anything previously investigated – or currently in development – by others. VRON-0200 has already achieved its first patients dosed, in their multi-national, first-in-humans study, with early clinical readouts expected in the first quarter of 2024.
In addition to the recent major clinical and scientific milestones achieved through its JV partner, Ocean continues to advance immunotherapies for lung, brain, and other cancers by targeting chitinase 3-like-1 expression (CHi3L1) and continues to progress additional development programs in fibrosis and for the treatment, and prevention, of malaria.
Anti-cancer Programs
Jack Elias MD, Ocean’s co-scientific founder, and his colleagues at Yale and Brown Universities recently published data showing the company’s cancer immunotherapy antibody candidate that targets CHi3L1. It has demonstrated effective tumor reduction against an aggressive subset of Non-Small Cell Lung Cancer with Epidermal Growth Factor Receptor mutations. By suppressing CHi3L1 activity, this treatment demonstrated a stunning ability to restore therapeutic sensitivity to current tyrosine kinase inhibitor therapies after resistance sets in, including the third-generation TKI, Osimertinib. In addition, recent studies have demonstrated up to 95% reduction in primary and metastatic tumor burden in mouse models of lung cancer.
“I am excited about these major milestones through our Joint Venture with Virion, and Ocean’s progress through our pipeline. These recent inflection points enhance the current and future potential value of our company, and we are excited for upcoming clinical data from the joint venture and other strategic activities for Ocean,” said Dr. Chirinjeev Kathuria, Ocean’s executive chairman and co-founder.