Market Performance Update
Anavex Q1 2026 Earnings: First Quarter Financial Results
First quarter fiscal 2026 showed improved financial metrics. Cash and cash equivalents reached $131.7 million at December 31, 2025. This compares to $102.6 million at September 30, 2025. Consequently, the company strengthened its balance sheet considerably. Research and development expenses totaled $4.7 million. This declined from $10.4 million in the comparable quarter of fiscal 2025. Similarly, general and administrative expenses fell to $2.1 million. This dropped from $3.1 million year-over-year. Net loss narrowed to $5.7 million. This improved from $12.1 million in the prior year quarter.
Quarterly Cash Position Trend
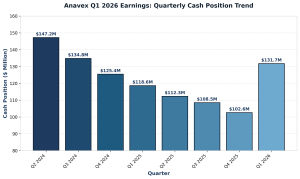
Anavex Q1 2026 Earnings: Quarterly Cash Position (2024-2026)
Clinical Pipeline Advances Multiple Programs
Anavex continues progressing its clinical pipeline. The lead candidate is oral blarcamesine for early Alzheimer’s disease. In Phase IIb/III trials, blarcamesine slowed clinical progression by 36.3% at week 48. Moreover, the pre-specified patient group showed 49.8% slowing. The drug demonstrated a favorable safety profile. Specifically, no neuroimaging adverse events occurred. Additionally, no deaths related to study drug were reported. Blarcamesine also slowed brain volume loss significantly.
CEO Christopher U. Missling, PhD highlighted the company’s mission. According to Missling, approximately 7.2 million people in the U.S. live with Alzheimer’s disease. Furthermore, about 7 million Europeans have the condition. Therefore, the market opportunity remains substantial. The company aims to develop oral, once-daily treatments. This approach offers convenience over injectable alternatives.
Operating Expenses Analysis
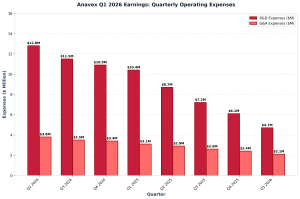
Anavex Q1 2026 Earnings: R&D and G&A Expense Trends
Regulatory Pathway Updates
The company received FDA Type C meeting feedback in January 2026. The FDA expressed interest in Anavex’s development plans. In fact, the agency showed a collaborative approach. The meeting discussed pathways to support a New Drug Application. Consequently, existing Phase IIb/III data will be submitted as requested. In Europe, the EMA review process continues. The CHMP previously adopted a negative opinion. However, Anavex requested re-examination in December 2025. A different rapporteur now leads the review process.
Recent Corporate Developments
Anavex announced several corporate milestones. In January 2026, Wolfgang Liedtke, MD PhD joined as Senior Vice President. He serves as Global Head of Neurology. Dr. Liedtke brings 25 years of CNS drug development experience. Previously, he chaired Neurology at Regeneron. He oversaw 45 clinical trials including 14 Phase 3 studies. Additionally, Anavex joined ACCESS-AD in January 2026. This European initiative aims to accelerate Alzheimer’s diagnostic adoption. The Innovative Health Initiative funds the program.
Precision Medicine Approach Shows Promise
Blarcamesine demonstrates enhanced efficacy in specific populations. The SIGMAR1 Wild Type population comprises about 70% of patients. In this group, treatment effects increased substantially. ADAS-Cog13 differences reached 49.8% improvement over placebo. CDR-SB showed 33.7% benefit in this population. The ABCLEAR3 precision medicine cohort showed even better results. Specifically, ADAS-Cog13 improved 84.7% versus placebo. This population represents about 50% of early Alzheimer’s patients.
Anavex Q1 2026 Earnings: Outlook and Milestones
Management outlined several expected milestones. The company anticipates EMA re-examination results in H1 2026. Initiation of a clinical prediction study is planned. A Parkinson’s disease imaging-focused trial may begin. Fragile X Phase 2/3 clinical trial initiation is expected. Moreover, publications on precision medicine findings are forthcoming. The company maintains broad IP protection through 2040. Operations remain funded for more than three years.
Anavex Q1 2026 Earnings: Key Takeaways
In summary, Anavex Q1 2026 earnings demonstrated improved financial discipline. Cash position increased 28% to $131.7 million. As a result, the company maintains over three years of runway. R&D expenses declined 55% year-over-year. This reflects efficient clinical program management. The blarcamesine Alzheimer’s program advances toward potential approval. Precision medicine approaches show promising clinical benefits. For more information, visit the Anavex Q1 2026 earnings investor relations page. Connect with the company on Anavex LinkedIn for the latest updates.