COVID-19 vaccine
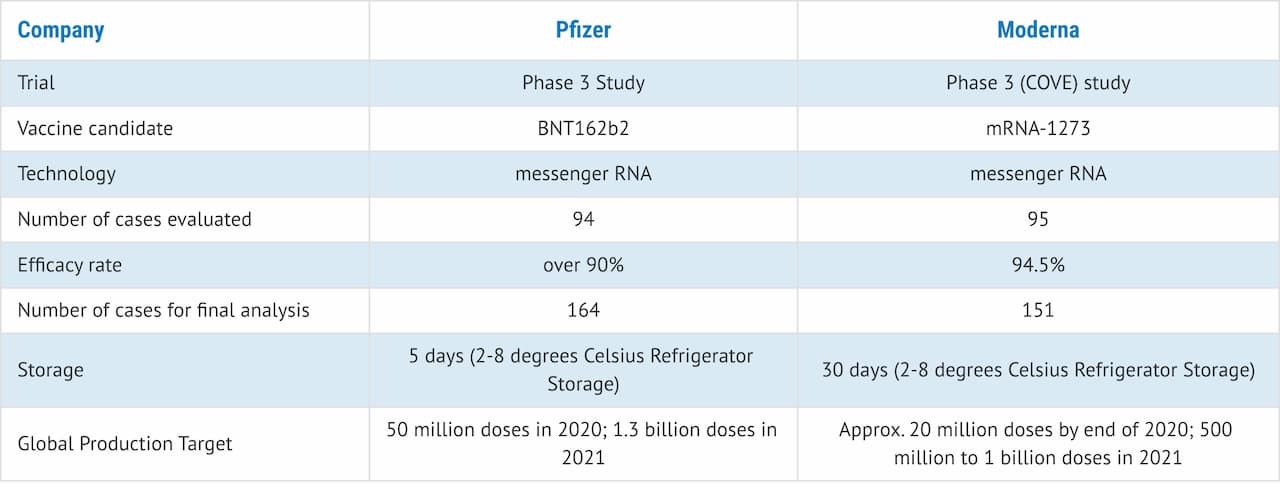
Moderna announced today that the Phase 3 study of mRNA-1273, its COVID-19 vaccine candidate, has displayed an efficacy rate of 94.5%. The COVE study, as it is called, has over 30,000 participants in the US. The interim analysis of the two-dose vaccine on 95 cases revealed no significant safety issues. The vaccine was well-tolerated in general and had only mild to moderate side-effects.
Based on this data, Moderna plans to apply for approval and clearance from the US FDA as well as regulatory agencies around the world over the coming weeks. The final analysis will be based on 151 cases. The study is being conducted in partnership with the National Institute of Allergy and Infectious Diseases (NIAID), and the Biomedical Advanced Research and Development Authority (BARDA). The trial results were reviewed by an independent research committee.
Moderna expects to have around 20 million vaccine doses ready by the end of 2020 and it is on track to manufacture 500 million to 1 billion doses worldwide in 2021.
In a separate release, Moderna said its COVID-19 vaccine candidate has a longer shelf life when stored at low temperatures. The vaccine is expected to stay stable at -20°C for up to six months in terms of shipping and long-term storage. This is equivalent to temperatures in most home or medical freezers.
At standard refrigerated conditions of 2° to 8°C, the vaccine can stay stable for up to 30 days within its six-month shelf life. This applies to most hospitals and pharmacies. The vaccine can stay at room temperature for up to 12 hours and it does not require dilution or special handling.
COVID-19 Vaccine – Moderna vs. Pfizer
Competitors
Apart from Moderna, several other pharmaceutical companies are actively involved in the development of a vaccine against COVID-19. Last week, Pfizer Inc. (NYSE: PFE) disclosed that its mRNA-based vaccine candidate, BNT162b2, displayed an efficacy rate of over 90% based on 94 cases. Pfizer and its partner BioNTech SE (NASDAQ: BNTX) are also working on getting approval from the FDA.
Other candidates in the race for a vaccine against COVID-19 include Johnson & Johnson (NYSE: JNJ), Novavax (NASDAQ: NVAX) and AstraZeneca (NASDAQ: AZN).
COVID-19
The COVID-19 pandemic has reached 54.5 million cases worldwide and caused 1.32 million deaths. In the US, the number of cases stand at 11.1 million with the number of deaths over 246,000.
Quarterly performance
For its most recent quarter, Moderna reported revenues of nearly $158 million which surpassed Street expectations but net loss widened to $0.59 per share, missing market expectations.
Click here to read the full transcript of Moderna Q3 2020 earnings conference call