During the pre-market trading session yesterday, LMNL stock climbed up by 75%. After opening at $20.00, shares of Liminal BioSciences pared the gains later in the day and turned to red by ending down 1.09% at $13.59%. The stock continued to bleed during the extended hours of yesterday’s trading session, which plunged more than 10%.

Q2 results
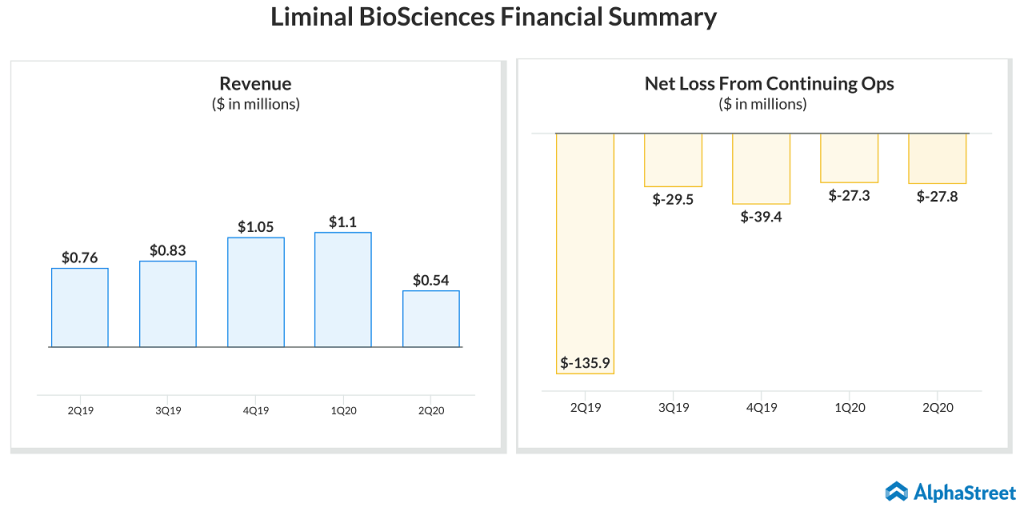
Liminal’s net loss from continuing operations in the second quarter reduced to $27.8 million from $135.9 million in the previous year due to its debt restructuring and decline in expenses. Revenue declined to $0.54 million in the second quarter from $0.8 million in the same period a year ago.
Reason for the buzz
On Sunday, the US FDA issued an emergency use authorization (EUA) for investigational convalescent plasma for the treatment of COVID-19 in hospitalized patients as part of the agency’s ongoing efforts to fight COVID-19. The EUA authorizes the distribution of COVID-19 convalescent plasma in the US and its administration by health care providers, as appropriate, to treat suspected or laboratory-confirmed COVID-19 hospitalized patients.
Liminal BioSciences is involved in the development of plasma-based therapeutics. The company believes its infrastructure and capabilities for collecting specialty plasma in the plasma collection centers in the US and Canada will be useful in contributing to the treatment of COVID-19. The FDA announcement triggered a positive momentum for LMNL stock during yesterday’s morning session. However, later in the day LMNL stock started trading in the negative territory.
When answering a question on plasma therapy during the World Health Organization’s briefing on COVID-19, the chief scientist of WHO, Dr. Soumya Swaminathan stated that convalescent plasma therapy has been effective in some and not in some diseases. In this method, plasma is collected from people who have recovered from COVID-19 and then it is used to transfuse into someone who is affected by the deadly disease. She added that convalescent plasma therapy is still an experimental therapy and not standardized therapy. All the hospitals don’t have the required medical facility and there is also lack of plasma donors.
What Liminal does
Liminal is a clinical-stage biopharmaceutical company focused on discovering, developing and commercializing novel treatments for patients suffering from diseases that have high unmet medical need, including those related to fibrosis in respiratory, liver and kidney diseases. Liminal BioSciences has active business operations in Canada, the United Kingdom and the United States.
Prometic Plasma Resources, a subsidiary of Liminal BioSciences, has joined the CoVIg19 Plasma Alliance to contribute to the acceleration of the development of a potential new therapy for COVID-19. Liminal’s American plasma collection center is located in Amherst, New York.
The company’s BLA with the FDA for Ryplazim, for the treatment of congenital plasminogen deficiency, is anticipated to be filed in the third quarter of 2020. Liminal expects PDUFA date for Ryplazim in Q1 2021 and expects potential monetization of Priority Review Voucher, if granted by FDA on successful Ryplazim BLA approval, in 2021.
Since Liminal got listed on Nasdaq in the fourth quarter of 2019, the majority of its active daily trading volume transitioned to Nasdaq and its shares got delisted from the Toronto Stock Exchange effective August 5.
Final verdict
On Sunday, President Trump said, “Today, I am pleased to make a truly historic announcement in our battle against China virus, that will save countless lives. The FDA has issued an emergency use authorization for a treatment known as convalescent plasma.” But the medical experts around the world are concerned that a push to give out the blood plasma could weaken the clinical trials needed to determine whether it actually works against COVID-19. As stated earlier, WHO also expressed its concerns on using convalescent plasma therapy for COVID-19.
Yesterday, the other COVID plasma stocks Sonnet Biotherapeutics (NASDAQ: SONN), T2 Biosystems (NASDAQ: TTOO) and Kamada (NASDAQ: KMDA) created a lot of buzz. by gaining 48%, 7% and 17%, respectively. However, retail investors can wait and watch whether Liminal Biosciences can produce any significant revenue from the plasma treatment and then make a decision. Because there could be no big revenue generation for Liminal as a result of the plasma treatment even if it works and treats the COVID-19.
DISCLAIMER: The article does not necessarily imply the views of AlphaStreet, and contains opinions of the author alone.