“
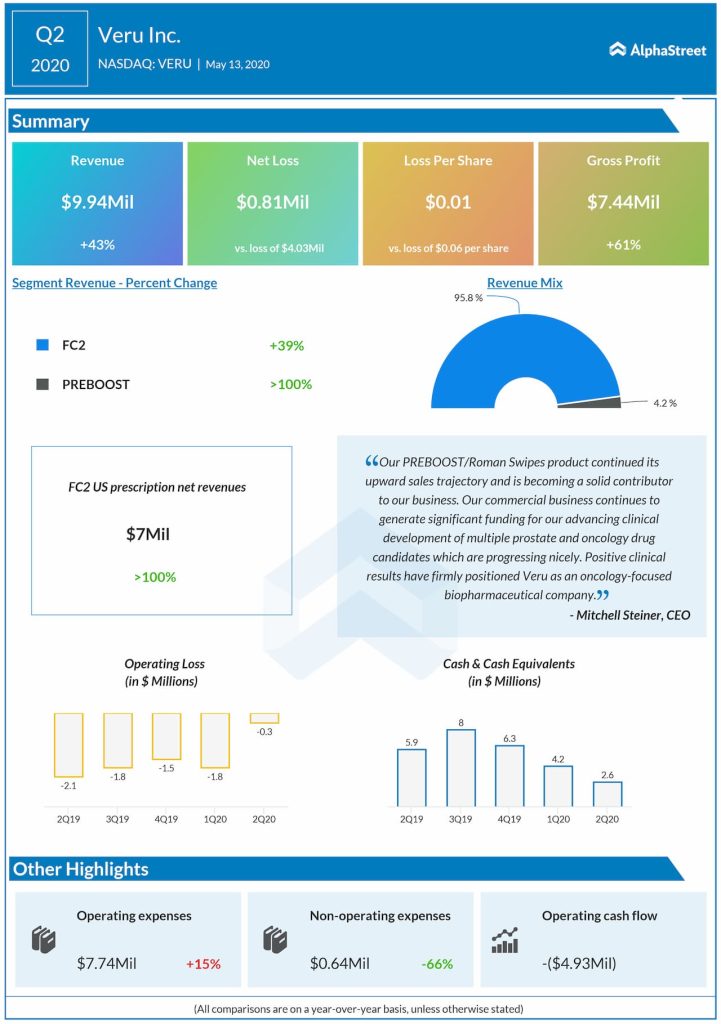
Veru Inc. (NASDAQ: VERU) reported its financial results for the quarter ended March 31, 2020, on Wednesday before the market opens. The company posted a narrower loss for Q2 as the substantial growth in prescription sales of FC2 drove the top-line higher. On Tuesday, Veru received FDA permission to initiate a phase 2 clinical trial […]
· May 13, 2020
Veru Inc. (NASDAQ: VERU) reported its financial results for the quarter ended March 31, 2020, on Wednesday before the market opens. The company posted a narrower loss for Q2 as the substantial growth in prescription sales of FC2 drove the top-line higher.
On Tuesday, Veru received FDA permission to initiate a phase 2 clinical trial to assess the efficacy of VERU-111 in combating COVID-19, the global pandemic disease caused by the novel coronavirus SARS-CoV-2. Veru has initiated the study and expects the first patient to be dosed within 2 weeks.