COVID-19 has been pretty disruptive on clinical-level biotech and pharmaceutical companies worldwide, especially those running on tight budgets. Clinical trials have been obstructed and dialogues with regulators have been delayed, in turn, leading to extended timelines for products. Meanwhile, ImmunoGen Inc (NASDAQ: IMGN) presented a different story when it reported Q1 earnings last week.
Although the results failed to surpass Wall Street projections, the Waltham, Massachusetts-based firm said its timelines and pipeline remained mostly unaffected by the pandemic, which offers enough respite to investors.
In a post-earnings interview, ImmunoGen CEO Mark Enyedy told AlphaStreet that the firm entered the lockdown phase with enough drug supply for all the existing clinical trial studies, including for Phase 3-level platinum-resistant ovarian cancer candidate mirvetuximab soravtansine, as well as IMGN632 and IMGC936. Immunogen will be filing the IND for IMGC936 later this quarter.
Tackling the pandemic crisis
The management added that there were no regulatory delays as well during the quarter, primarily because these are studies for diseases with unmet needs. Meanwhile, on the disruptions in the activity side, Chief Medical Officer Anna Berkenblit said:
“Both SORAYA and MIRASOL are really heavily in the activation phase right now. It’s really just a minority of sites that are having some delays in activation and it’s mostly due to leaner staffing model, people working from home or fewer people on site to move paperwork through. But for the most part, we are continuing to activate our sites. We’re doing remote site initiation visits and getting folks up and running.”
ADVERTISEMENT
Monotherapy vs. combination cohorts
Berkenblit cleared confusion surrounding the company’s strategy on monotherapy approvals with regards to mirvetuximab, following encouraging results from the merv + Avastin combination cohorts. She said:
“It does not change our initial fast-to-market strategy for monotherapy approval. What it does is, it allows us with these two cohorts treated with mirv + Avastin to have a robust dataset to support compendia listing, that we should be able to get shortly after we get our initial monotherapy approval.”
She pointed out that it offers physicians a larger data set and more options for using the drug.
Money matters
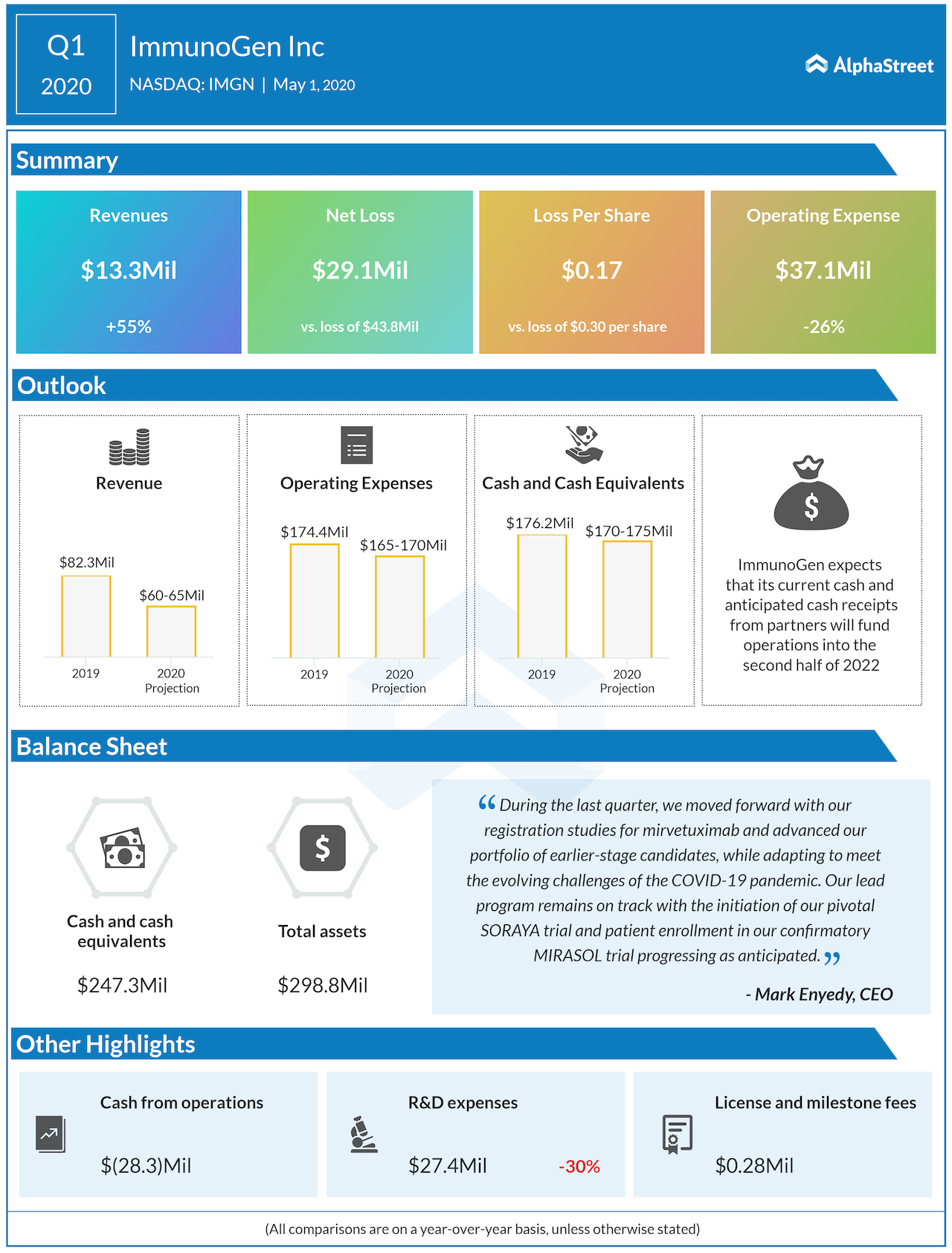
At the end of the first quarter, ImmunoGen had cash and cash equivalents of $247.3 million, partly due to a $98 million follow-on offering in January. The company expects the cash balances to be sufficient until the second half of 2022, well past the expected timeline for approval from SORAYA in the first half of 2022.
CEO Mark Enyedy asserted that the follow-on
offering in January was done to have sufficient cash buffer following the
readout of SORAYA and also anticipating the readout of MIRASOL.
[irp posts=”58315″]
Meanwhile, on the revenue side, the CEO declined from providing specifics on the discussions happening with potential royalty partners. Kadcyla royalties have been fully monetized by last year, with total proceeds of $265 million.
Meanwhile, Enyedy once again stressed upon the China agreement talks, which were earlier hinted during the earnings conference call.
Takeaways from FORWARD-1
Following the failure of the FORWARD-1 trial, the company was focused on figuring out its shortcomings, restructuring operations to reduce costs, and prioritizing programs that had the highest probability of success. In the words of Enyedy:
“It was very clear to us that the drug was active in patients with high levels of fully receptor alpha. The question was how to capitalize on that observation. And that was the important work that we did in the background for 2019 to work closely with the FDA to agree on a path to accelerated approval, the result of which is the design of SORAYA that we’re carrying out.”
ADVERTISEMENT
Speaking of the early-stage portfolio, Berkenblit said the company would share pre-clinical data from IMGN151 showing the benefit of biparatopic engineering at the AACR (American Association of Cancer Research) conference in June. Asked about the significance of IMGN151, the Chief Medical Officer stated:
“This opens up a whole variety of additional tumors that are Fr alpha positive, but express lower levels of Fr alpha than are currently tractable with mirvetuximab. So we are talking about an additional potentially 40-60% of ovarian cancer patients and additional endometrial cancer patients, triple-negative breast cancer and lung cancer.”
(Written by Arjun Vijay)
For more insights on ImmunoGen, read the latest earnings call transcript here.
[irp posts=”57665″]
