Palatin-AMAG Vyleesi agreement
On January 9, 2020, AMAG announced plans to divest Vyleesi and Intrarosa, both women’s healthcare products. While AMAG indicated that it has received preliminary expressions of interest to acquire or sublicense rights to these products, there have been no public disclosures of any potential licensees of AMAG’s rights to Vyleesi in North America. When AMAG reported its first quarter earnings on May 11, just a day before Palatan’s quarterly reporting, AMAG’s CEO Scott Myers said,
“As we previously mentioned, we are progressing the Intrarosa and Vyleesi divestitures and we will provide an update by the end of the second quarter.”
Palatin receives a royalty on sales of Vyleesi by the licensees. AMAG is currently selling Vyleesi in the U.S., and Fosun and Kwangdong have licenses to sell Vyleesi in China and Korea, respectively. Due to the early commercial stage of Vyleesi and the sales and marketing strategy of AMAG, including no charge for the first Vyleesi prescription, AMAG has not generated positive net sales in the three months ended March 31, 2020, which resulted in no royalties to Palatin during this period.
[irp posts=”61025″]
Clinical pipeline
Palatin’s clinical trial for the dry eye disease is in Phase 2 now and the company expects Phase 3 studies to start in the first half of 2021. Clinical trials for noninfectious uveitis are now scheduled to begin in the first and second half of 2021. The Phase 2 clinical study in ulcerative colitis patients is scheduled to start in the first half of 2021 with data readout in the second half of 2022, which represents a two-quarter delay due to the pandemic.
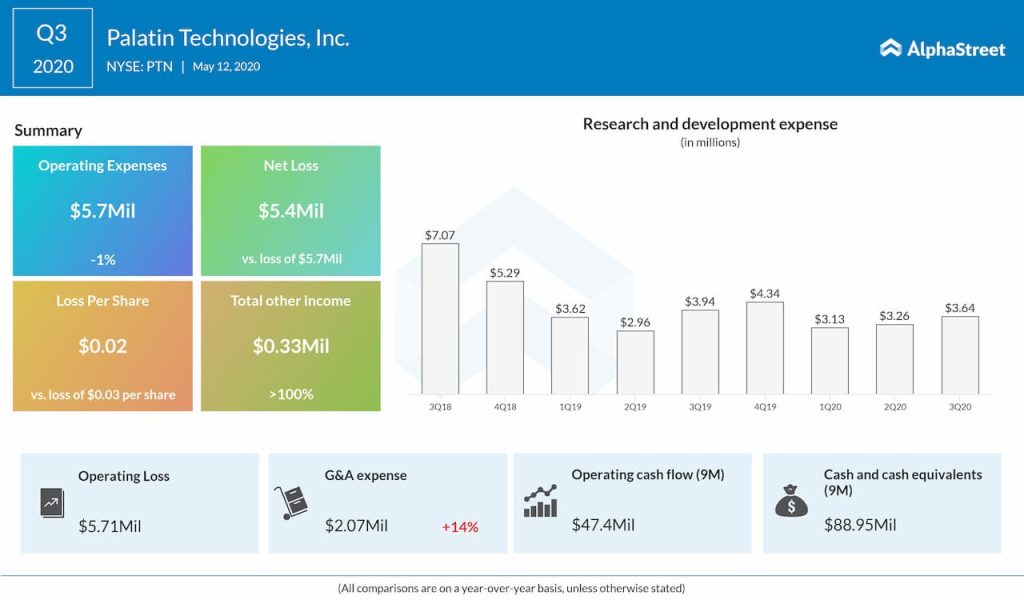
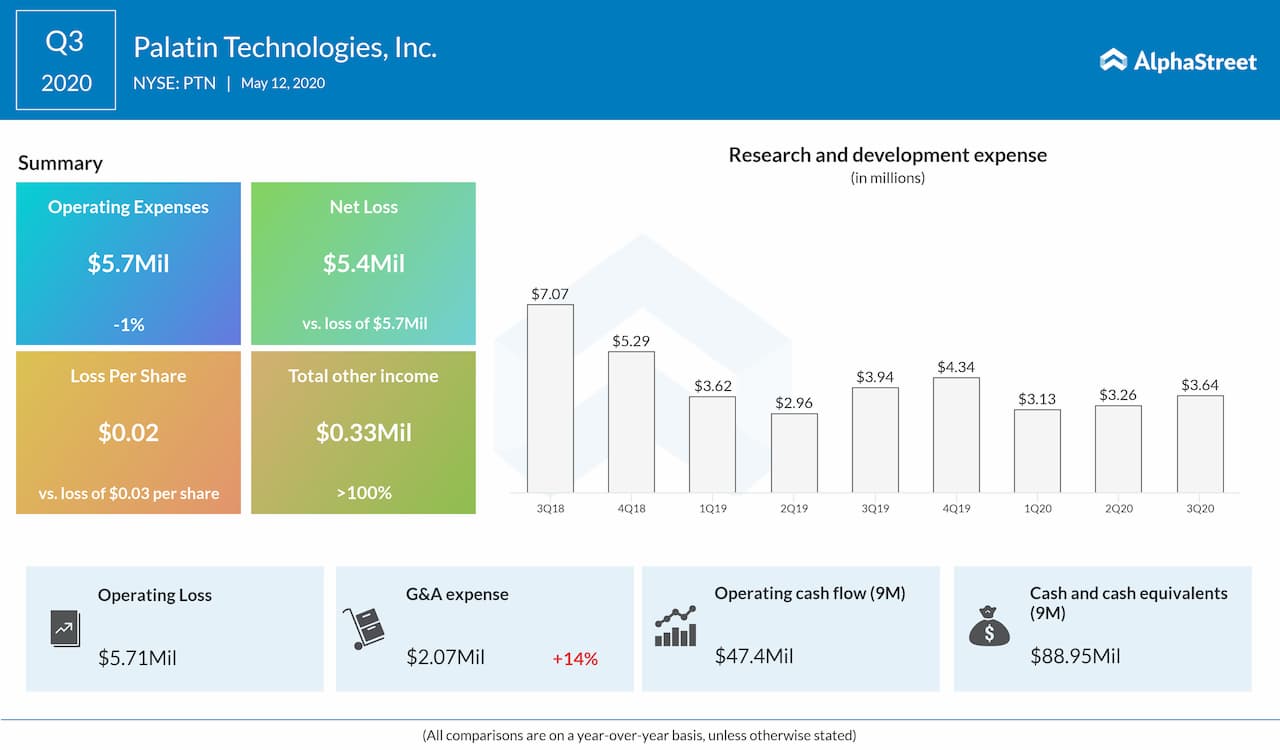
Q3 2020 results
Palatin reported a loss of $5.4 million or $0.02 per share for the quarter ended March 31, 2020, compared to a net loss of $5.7 million or $0.03 per share for the same period in 2019. As already stated, the company didn’t record any revenue in the third quarter. With cash and cash equivalents of $88.9 million as of March 31, 2020, the Cranbury, New Jersey-based firm believes that existing capital resources will be adequate to fund its planned operations through at least March 31, 2022.
Vyleesi’s future
Though sales of Vyleesi have been adversely affected by the COVID-19 pandemic, AMAG’s divestiture also hurt Vylessi’s sales. Palatin continues to discuss Vyleesi collaborations for the regions outside the currently licensed territories of North America, China and Korea and anticipates executing multiple agreements during the second half of this year and during calendar 2021. Until the company gets a new partner in North America, the value of Vyleesi is expected to come down.
[irp posts=”61157″]
As per the agreement, AMAG has to make contingent payments of up to $300 million of aggregate sales milestone payments to Palatin upon the achievement of certain annual net sales milestones over the course of the license. As AMAG is now in the process of divesting Vyleesi, it’s not clear how much compensation will Palatin seek from AMAG for breaking the agreement. Also, if Palatin obtains all rights to Vyleesi from AMAG, as a result of the termination of the license agreement, it will be tough for Palatin to establish sales and marketing, contract manufacturing, and distribution, which will be expensive and time consuming.
When an analyst asked in the earnings call whether Palatin had entered into legal arena with regard to the potential breach of contract, CEO Carl Spana said,
“No, we haven’t. Our most favored outcome would be that the divestiture of Vyleesi occurs in a manner that is best with the product without any recourse to legal actions. We’re going to protect our rights to ensure that Vyleesi winds up in a home with a company committed to realizing that value.”