The patent war between Amarin (NASDAQ: AMRN) and the generic companies for cardiovascular drug Vascepa continues to be a headwind for Amarin. Shares of the clinical biotechnology company were shattered at the end of March after the Nevada Court ruled in favor of two generic companies in the patent litigation case related to the generic versions of Vascepa. After losing two-thirds of its value and plunging to a new 52-week low ($3.95) on the next day after the court ruling, AMRN stock is yet to reach the double-digit mark.

Vascepa overview
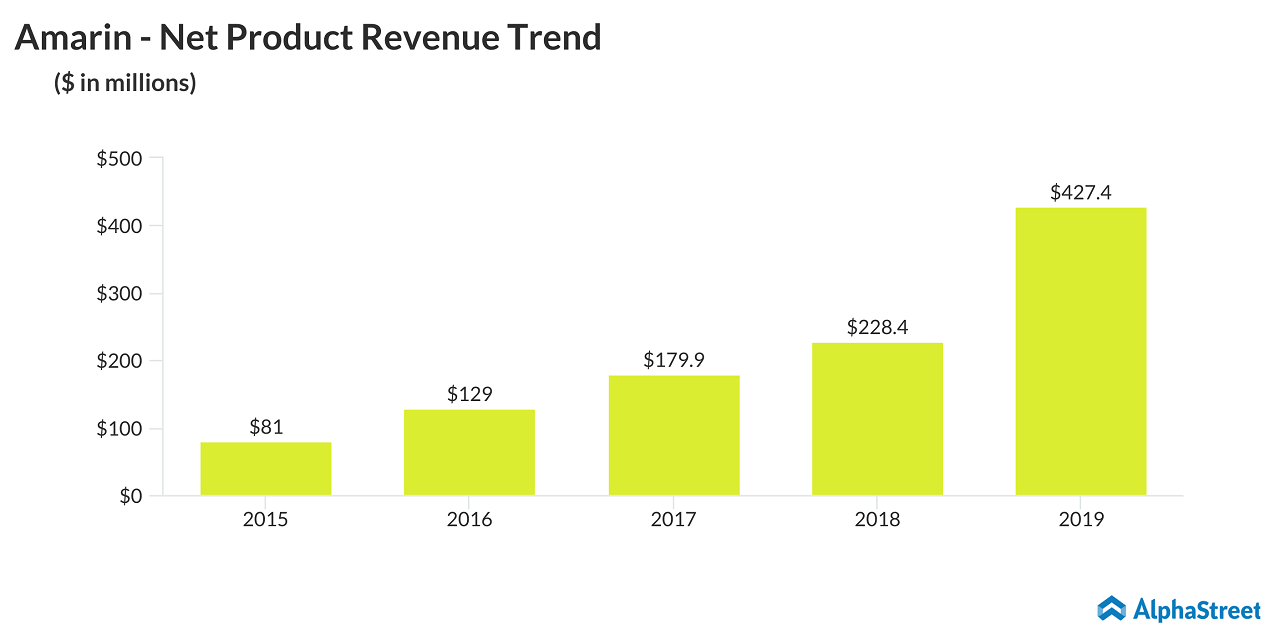
Amarin’s lead product Vascepa was first approved by the FDA in July 2012 for reducing triglyceride in severely affected adult patients. In January 2013, the company began selling and marketing 1-gram size Vascepa capsules in the US, and in October 2016, introduced a smaller 0.5-gram capsule size. In December 2019, the FDA approved a new indication and label expansion for Vascepa based on the results of the company’s cardiovascular outcomes trial, REDUCE-IT.
Vascepa is now available by prescription in the US, Canada,
Lebanon and the United Arab Emirates.
The Dublin, Ireland-based company has increased its focus on expanding
Vascepa to major markets outside the US. Amarin has strategic partnerships to
develop and commercialize Vascepa in select territories and the company is
pursuing additional regulatory approvals for its cardiovascular drug in China,
the European Union and the Middle East.
Litigation
Beginning in late 2017, Amarin was involved in litigation
against Dr. Reddy’s Laboratories and Hikma Pharmaceuticals USA in the U.S.
District Court for the District of Nevada. The company sought an order from the
court injunctioning these companies from marketing the generic versions of
Vascepa. On March 30, 2020, the Nevada Court issued its ruling in favor of Dr.
Reddy’s and Hikma. Amarin has appealed this verdict.
Meanwhile, Hikma received a go-ahead signal for its generic version of Vascepa from the FDA last month. If a generic version of Vascepa is launched during the appeal process and Amarin wins the appeal, the generic company that launched will be at risk of paying potentially large damages to Amarin. But if Amarin loses the appeal, a generic company would be able to immediately launch the generic version of Vascepa.
[irp posts=”55672″]
Last Tuesday, Amarin struck a deal with generic maker
Apotex. As per the agreement, Apotex may not sell a generic version of Vascepa
in the US until August 9, 2029. It’s worth noting that Amarin entered into a
similar deal with Teva Pharmaceuticals USA in 2018. Again, this agreement will
be valid only if Amarin wins the appeal in the Nevada Court.
During the first quarter earnings call, Amarin stated that it would file the initial appeal brief on May 12, followed by the defendants’ filing a responsive brief on June 16. Amarin is set to file its reply brief on June 26.
Amarin expects the Federal Circuit to hold a hearing in the first week of September or October 2020. The ruling on the appeal is expected before the end of 2020 or in early 2021. If Amarin wins the appeal it would spend more on marketing Vascepa in the US and consider launching its own generic.
Analysts’ opinion
Most of the analysts who are covering Amarin either recommend buying or holding the stock. With COVID-19 resulting in a sluggish sales environment for Vascepa, market watchers expect Amarin to remain in a range bound until we get closer to the court hearing at the end of 2020.
[irp posts=”59568″]